Activation Energy Of Forward Reaction
61 Activation Energy and Temperature Dependence
LumenLearning
Activation Energy
Activation energy is the energy required for a reaction to occur, and determines its charge per unit.
LEARNING OBJECTIVES
Discuss the concept of activation energy
Fundamental TAKEAWAYS
Fundamental Points
- Reactions require an input of free energy to initiate the reaction; this is called the activation energy (EA).
- Activation energy is the amount of energy required to achieve the transition state.
- The source of the activation energy needed to push reactions forward is typically heat energy from the surroundings.
- For cellular reactions to occur fast enough over short time scales, their activation energies are lowered by molecules chosen catalysts.
- Enzymes are catalysts.
Key Terms
- activation energy: The minimum energy required for a reaction to occur.
- catalysis: The increase in the rate of a chemical reaction by lowering its activation energy.
- transition state: An intermediate state during a chemical reaction that has a higher energy than the reactants or the products.
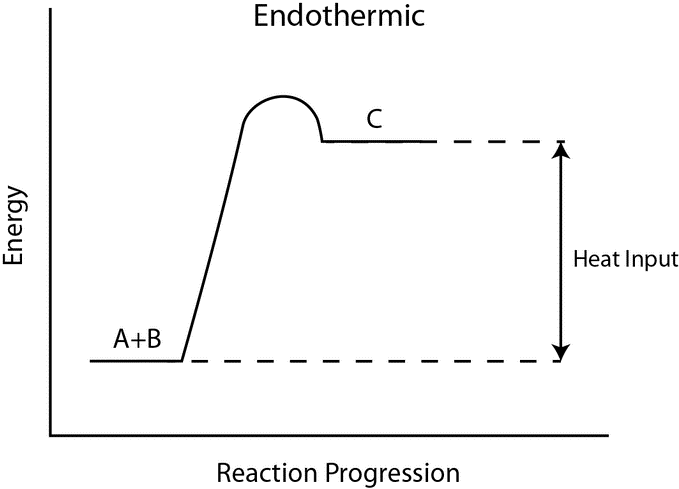
Many chemical reactions, and well-nigh all biochemical reactions do not occur spontaneously and must have an initial input of energy (called the activation free energy) to get started. Activation energy must exist considered when analyzing both endothermic and exothermic reactions. Exothermic reactions take a internet release of energy, simply they still crave a modest amount of energy input before they can proceed with their energy-releasing steps. This pocket-sized amount of energy input necessary for all chemical reactions to occur is called the activation nenergy (or free energy of activation) and is abbreviated EA.
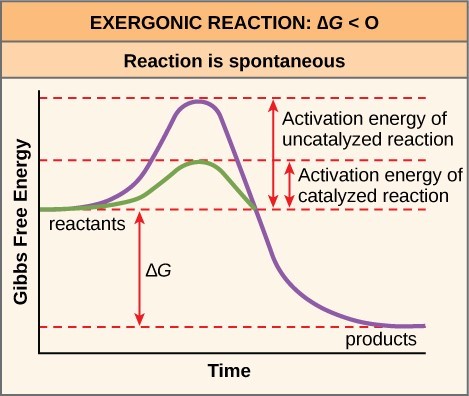
Activation Free energy in Chemical Reactions
Why would an energy-releasing, negative ∆G reaction actually crave some energy to proceed? The reason lies in the steps that take place during a chemical reaction. During chemical reactions, certain chemical bonds are broken and new ones are formed. For example, when a glucose molecule is broken downward, bonds between the carbon atoms of the molecule are broken. Since these are energy-storing bonds, they release energy when broken. Nonetheless, to get them into a state that allows the bonds to break, the molecule must be somewhat contorted. A small energy input is required to achieve this contorted state, which is called thetransition state: it is a high-energy, unstable state. For this reason, reactant molecules don't last long in their transition state, merely very chop-chop go along to the next steps of the chemical reaction.
Cells will at times couple an exothermic reaction ([latex]\Delta \text{G} < 0[/latex]) with endothermic reactions ([latex]\Delta \text{Thou} > 0[/latex]), allowing them to proceed. This spontaneous shift from ane reaction to some other is chosen free energy coupling. The gratuitous energy released from the exothermic reaction is absorbed by the endothermic reaction. One example of energy coupling using ATP involves a transmembrane ion pump that is extremely important for cellular role.
Free Energy Diagrams
Gratuitous free energy diagrams illustrate the free energy profiles for a given reaction. Whether the reaction is exothermic ([latex]\Delta \text{Chiliad} < 0[/latex]) or endothermic ([latex]\Delta \text{1000} > 0[/latex]) determines whether the products in the diagram volition exist at a lower or higher free energy land than the reactants. Nevertheless, the measure of the activation energy is independentof the reaction'southward [latex]\Delta G[/latex]. In other words, at a given temperature, the activation energy depends on the nature of the chemical transformation that takes place, but not on the relative energy land of the reactants and products.
Although the prototype in a higher place discusses the concept of activation free energy within the context of the exothermic forwards reaction, the same principles apply to the reverse reaction, which must be endothermic. Notice that the activation free energy for the reverse reaction is larger than for the forrad reaction.
The source of the activation energy needed to push reactions forward is typically heat energy from the surround. Heat energy (the full bond energy of reactants or products in a chemical reaction) speeds up the move of molecules, increasing the frequency and force with which they collide. It also moves atoms and bonds within the molecule slightly, helping them reach their transition state. For this reason, heating up a system will cause chemic reactants within that system to react more than frequently. Increasing the pressure on a system has the same effect. In one case reactants have captivated enough heat energy from their environment to reach the transition land, the reaction volition proceed.
The activation free energy of a particular reaction determines the charge per unit at which it volition go on. The higher the activation energy, the slower the chemical reaction volition be. The case of iron rusting illustrates an inherently slow reaction. This reaction occurs slowly over time considering of its high EA. Additionally, the burning of many fuels, which is strongly exothermic, will accept place at a negligible rate unless their activation energy is overcome by sufficient heat from a spark. Once they begin to burn down, nonetheless, the chemical reactions release enough heat to continue the burning process, supplying the activation free energy for surrounding fuel molecules.
Like these reactions outside of cells, the activation energy for almost cellular reactions is too high for heat free energy to overcome at efficient rates. In other words, in order for important cellular reactions to occur at significant rates (number of reactions per unit fourth dimension), their activation energies must be lowered; this is referred to as catalysis. This is a very good thing as far equally living cells are concerned. Important macromolecules, such as proteins, Deoxyribonucleic acid, and RNA, store considerable energy, and their breakdown is exothermic. If cellular temperatures alone provided enough heat free energy for these exothermic reactions to overcome their activation barriers, the essential components of a cell would disintegrate.
The Arrhenius Equation
The Arrhenius equations relates the rate of a chemical reaction to the magnitude of the activation energy:
[latex]\text{thousand} = \text{Ae}^{\text{Eastward}_\text{a}\text{/RT}[/latex]
where
- k is the reaction charge per unit coefficient or constant
- A is the frequency factor of the reaction. It is determined experimentally.
- R is the Universal Gas constant
- T is the temperature in Kelvin
The Standoff Theory
Collision theory provides a qualitative explanation of chemic reactions and the rates at which they occur, appealing to the principle that molecules must collide to react.
LEARNING OBJECTIVES
Discuss the role of activation energy, collisions, and molecular orientation in collision theory
Primal TAKEAWAYS
Fundamental Points
- Molecules must collide in order to react.
- In order to effectively initiate a reaction, collisions must be sufficiently energetic ( kinetic free energy ) to break chemic bonds; this energy is known as the activation energy.
- As the temperature rises, molecules motion faster and collide more than vigorously, greatly increasing the likelihood of bond breakage upon collision.
Primal Terms
- activation energy: The minimum energy with which reactants must collide in club for a reaction to occur.
Standoff Theory provides a qualitative explanation of chemical reactions and the rates at which they occur. A basic principal of collision theory is that, in order to react, molecules must collide. This fundamental dominion guides whatsoever analysis of an ordinary reaction mechanism.
Consider the uncomplicated bimolecular reaction: [latex]\text{A + B} \rightarrow \text{products}[/latex]
If the 2 molecules A and B are to react, they must come into contact with sufficient force so that chemical bonds break. Nosotros call such an encounter a collision. If both A and B are gases, the frequency of collisions between A and B volition exist proportional to the concentration of each gas. If we double the concentration of A, the frequency of A-B collisions will double, and doubling the concentration of B will accept the same issue. Therefore, according to collision theory, the rate at which molecules collide will accept an touch on on the overall reaction rate.
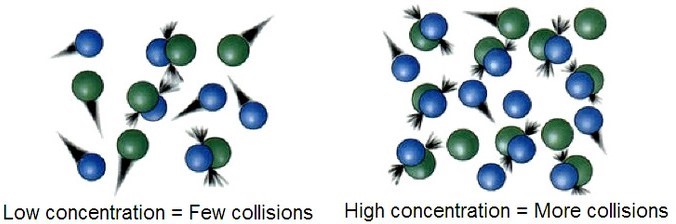
Activation Energy and Temperature
When ii billiard balls collide, they simply bounciness off of one other. This is also the most likely effect when two molecules, A and B, come into contact: they bounce off ane another, completely unchanged and unaffected. In lodge for a collision to existsuccessful past resulting in a chemical reaction, A and B must collide with sufficient free energy tobreak chemical bonds. This is because in any chemical reaction, chemic bonds in the reactants are broken, and new bonds in the products are formed. Therefore, in order to effectively initiate a reaction, the reactants must be moving fast plenty (with enough kinetic free energy) so that they collide with sufficient force for bonds to suspension. This minimum energy with which molecules must be moving in club for a collision to issue in a chemical reaction is known every bit theactivation energy.
As we know from the kinetic theory of gases, the kinetic energy of a gas is directly proportional to temperature. As temperature increases, molecules proceeds energy and motility faster and faster. Therefore, the greater the temperature, the higher the probability that molecules will be moving with the necessary activation free energy for a reaction to occur upon standoff.
Molecular Orientation and Constructive Collisions
Even if two molecules collide with sufficient activation energy, in that location is no guarantee that the collision will exist successful. In fact, the collision theory says that not every standoff is successful, even if molecules are moving with plenty energy. The reason for this is considering molecules too need to collide with the rightorientation, so that the proper atoms line up with one another, and bonds tin break and re-form in the necessary way. For instance, in the gas- phase reaction of dinitrogen oxide with nitric oxide, the oxygen stop of NiiO must hit the nitrogen end of NO; if either molecule is not lined up correctly, no reaction volition occur upon their standoff, regardless of how much energy they have. However, considering molecules in the liquid and gas stage are in constant, random motion, there is always the probability that 2 molecules will collide in just the right mode for them to react.
Of course, the more critical this orientational requirement is, similar it is for larger or more complex molecules, the fewer collisions there volition exist that will beconstructive. Aneffective collision is defined equally one in which molecules collide with sufficient energyand proper orientation, then that a reaction occurs.
Conclusion
According to the collision theory, the following criteria must be met in society for a chemical reaction to occur:
- Molecules must collide with sufficient energy, known equally the activation free energy, so that chemical bonds can suspension.
- Molecules must collide with the proper orientation.
- A standoff that meets these two criteria, and that results in a chemical reaction, is known equally a successful standoff or an effective collision.
Collision theory explanation: Standoff theory provides an caption for how particles interact to cause a reaction and the germination of new products.
Factors that Affect Reaction Charge per unit
The charge per unit of a chemical reaction depends on factors that affect whether reactants tin can collide with sufficient energy for reaction to occur.
LEARNING OBJECTIVES
Explain how concentration, surface area, pressure, temperature, and the addition of catalysts bear upon reaction rate
KEY TAKEAWAYS
Key Points
- When the concentrations of the reactants are raised, the reaction gain more than chop-chop. This is due to an increase in the number of molecules that take the minimum required energy. For gases, increasing pressure has the same effect as increasing concentration.
- When solids and liquids react, increasing the surface expanse of the solid will increase the reaction rate. A decrease in particle size causes an increment in the solid's total surface expanse.
- Raising the reaction temperature past ten °C tin double or triple the reaction rate. This is due to an increase in the number of particles that have the minimum free energy required. The reaction charge per unit decreases with a decrease in temperature.
- Catalysts can lower the activation energy and increase the reaction rate without being consumed in the reaction.
- Differences in the inherent structures of reactants can pb to differences in reaction rates. Molecules joined by stronger bonds will take lower reaction rates than volition molecules joined past weaker bonds, due to the increased corporeality of energy required to suspension the stronger bonds.
Key Terms
- goad: A substance that increases the rate of a chemical reaction without beingness consumed in the process.
- activation energy: The minimum amount of energy that molecules must have in order for a reaction to occur upon collision.
Reactant Concentrations
Raising the concentrations of reactants makes the reaction happen at a faster rate. For a chemical reaction to occur, there must exist a certain number of molecules with energies equal to or greater than the activation free energy. With an increment in concentration, the number of molecules with the minimum required energy will increase, and therefore the rate of the reaction will increase. For example, if ane in a meg particles has sufficient activation energy, so out of 100 one thousand thousand particles, simply 100 will react. However, if yous have 200 million of those particles within the same volume, then 200 of them react. By doubling the concentration, the charge per unit of reaction has doubled too.
https://lab.concord.org/embeddable.html#interactives/sam/chemical-reactions/2-concentration-and-reaction-charge per unit.json
Interactive: Concentration and Reaction Rate: In this model, two atoms tin can form a bail to brand a molecule. Experiment with irresolute the concentration of the atoms in order to see how this affects the reaction rate (the speed at which the reaction occurs).
Surface Area
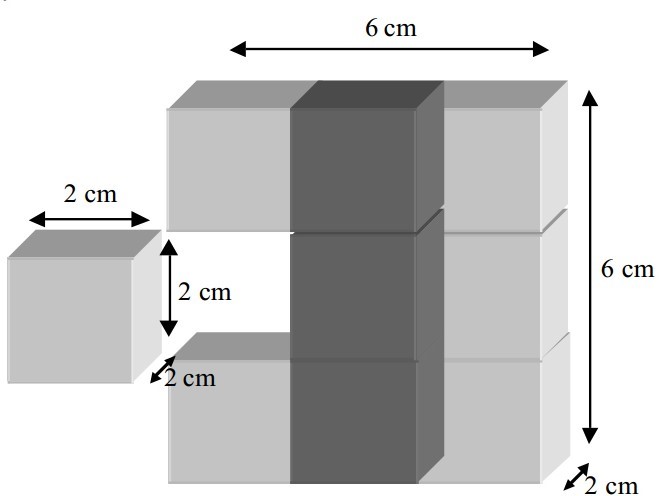
In a reaction between a solid and a liquid, the surface area of the solid will ultimately bear upon how fast the reaction occurs. This is because the liquid and the solid can bump into each other only at the liquid-solid interface, which is on the surface of the solid. The solid molecules trapped within the body of the solid cannot react. Therefore, increasing the surface area of the solid will expose more solid molecules to the liquid, which allows for a faster reaction.
For instance, consider a 6 x vi x 2 inch brick. The area of the exposed surfaces of the brick is [latex]four(6 \times 2) + 2(half dozen \times six) = 120 \text{ cm}^2[/latex]. When the brick is dismantled into 9 smaller cubes, nevertheless, each cube has a surface expanse of [latex]6(2 \times ii) = 24 \text{ cm}^ii[/latex], so the total surface area of the nine cubes is [latex]nine \times 24 = 216 \text{ cm}^2[/latex].
This shows that the full exposed expanse will increase when a larger body is divided into smaller pieces. Therefore, since a reaction takes place on the surface of a substance, increasing the surface expanse should increment the quantity of the substance that is available to react, and will thus increment the rate of the reaction as well.
Increasing the pressure level for a reaction involving gases will increase the rate of reaction. Equally you increase the pressure of a gas, you decrease its volume (PV=nRT; P and V are inversely related), while the number of particles (n) remains unchanged. Therefore, increasing pressure level increases the concentration of the gas (n/V), and ensures that the gas molecules collide more frequently. Keep in mind this logic only works for gases, which are highly compressible; changing the pressure for a reaction that involves only solids or liquids has no effect on the reaction charge per unit.
Temperature
It has been observed experimentally that a rise of 10 °C in temperature usually doubles or triples the speed of a reaction between molecules. The minimum energy needed for a reaction to keep, known every bit the activation energy, stays the same with increasing temperature. However, the average increase in particle kinetic energy caused past the absorbed rut means that a greater proportion of the reactant molecules now have the minimum energy necessary to collide and react. An increase in temperature causes a rise in the energy levels of the molecules involved in the reaction, and then the rate of the reaction increases. Similarly, the rate of reaction will decrease with a subtract in temperature.
https://lab.concord.org/embeddable.html#interactives/sam/chemical-reactions/iii-temperature-and-reaction-charge per unit.json
Interactive: Temperature and Reaction Rate: Explore the function of temperature on reaction rate. Note: In this model any rut generated by the reaction itself is removed, keeping the temperature abiding in guild to isolate the effect of environmental temperature on the rate of reaction.
Presence or Absence of a Catalyst
Catalysts are substances that increase reaction charge per unit by lowering the activation energy needed for the reaction to occur. A catalyst is not destroyed or changed during a reaction, and so it can be used again. For example, at ordinary conditions, H2 and O2 do non combine. However, they do combine in the presence of a small quantity of platinum, which acts as a catalyst, and the reaction then occurs speedily.
Nature of the Reactants
Substances differ markedly in the rates at which they undergo chemical change. The differences in reactivity between reactions may exist attributed to the different structures of the materials involved; for example, whether the substances are in solution or in the solid state matters. Some other gene has to do with the relative bond strengths inside the molecules of the reactants. For example, a reaction betwixt molecules with atoms that are bonded by strong covalent bonds volition take place at a slower rate than would a reaction between molecules with atoms that are bonded by weak covalent bonds. This is due to the fact that information technology takes more energy to break the bonds of the strongly bonded molecules.
LICENSES AND ATTRIBUTIONS
CC LICENSED CONTENT, SHARED PREVIOUSLY
- Curation and Revision.Provided by: Boundless.com.License:CC BY-SA: Attribution-ShareAlike
CC LICENSED CONTENT, SPECIFIC ATTRIBUTION
- OpenStax College, Biology. October 16, 2013.Provided past: OpenStax CNX.Located at: http://cnx.org/content/m44425/latest/?drove=col11448/latest.License:CC Past: Attribution
- catalysis.Provided past: Wiktionary.Located at: http://en.wiktionary.org/wiki/catalysis.License:CC BY-SA: Attribution-ShareAlike
- OpenStax, Biology. September 29, 2015.Provided past: OpenStax CNX.Located at: http://cnx.org/contents/185cbf87-c72e-48f5-b51e-f14f21b5eabd@ix.87.License:CC BY: Attribution
- activation energy.Provided by: Wiktionary.Located at: http://en.wiktionary.org/wiki/activation_energy.License:CC BY-SA: Attribution-ShareAlike
- transition state.Provided by: Wiktionary.Located at: http://en.wiktionary.org/wiki/transition_state.License:CC By-SA: Attribution-ShareAlike
- Endothermic Reaction.Provided past: Wikimedia.Located at: http://commons.wikimedia.org/wiki/File:Endothermic_Reaction.png.License:CC BY-SA: Attribution-ShareAlike
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. Oct 16, 2013.Provided by: OpenStax CNX.Located at: http://cnx.org/content/m44425/latest/Figure_06_03_04.jpg.License:CC BY: Attribution
- Activation energy, Arrhenius law.Provided by: Steve Lower'southward Website.Located at: http://world wide web.chem1.com/acad/webtext/dynamics/dynamics-iii.html.License:CC Past-SA: Attribution-ShareAlike
- Collision theory.Provided past: Wikipedia.Located at: http://en.wikipedia.org/wiki/Collision_theory.License:CC BY-SA: Attribution-ShareAlike
- activation free energy.Provided by: Wiktionary.Located at: http://en.wiktionary.org/wiki/activation_energy.License:CC BY-SA: Attribution-ShareAlike
- collision theory.Provided by: Wiktionary.Located at: http://en.wiktionary.org/wiki/collision_theory.License:CC By-SA: Attribution-ShareAlike
- Endothermic Reaction.Provided past: Wikimedia.Located at: http://eatables.wikimedia.org/wiki/File:Endothermic_Reaction.png.License:CC BY-SA: Attribution-ShareAlike
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. October sixteen, 2013.Provided by: OpenStax CNX.Located at: http://cnx.org/content/m44425/latest/Figure_06_03_04.jpg.License:CC BY: Attribution
- Collision theory caption.Located at: http://world wide web.youtube.com/watch?5=mBTSwJnZ6Sk.License:Public Domain: No Known Copyright.License Terms: Standard YouTube license
- Molecular-collisions.Provided by: Wikipedia.Located at: http://en.wikipedia.org/wiki/File:Molecular-collisions.jpg.License:CC Past-SA: Attribution-ShareAlike
- Provided by: African Virtual University.Located at: http://oer.avu.org/bitstream/handle/123456789/43/Chemistry%202%twenty-%20Introductory%20General.pdf?sequence=6.License:CC By: Attribution
- catalyst.Provided past: Wiktionary.Located at: http://en.wiktionary.org/wiki/catalyst.License:CC BY-SA: Attribution-ShareAlike
- concentration.Provided by: Wiktionary.Located at: http://en.wiktionary.org/wiki/concentration.License:CC Past-SA: Attribution-ShareAlike
- activation free energy.Provided past: Wiktionary.Located at: http://en.wiktionary.org/wiki/activation_energy.License:CC By-SA: Attribution-ShareAlike
- Endothermic Reaction.Provided by: Wikimedia.Located at: http://commons.wikimedia.org/wiki/File:Endothermic_Reaction.png.License:CC BY-SA: Attribution-ShareAlike
- OpenStax College, Potential, Kinetic, Free, and Activation Energy. October 16, 2013.Provided by: OpenStax CNX.Located at: http://cnx.org/content/m44425/latest/Figure_06_03_04.jpg.License:CC BY: Attribution
- Standoff theory explanation.Located at: http://www.youtube.com/watch?v=mBTSwJnZ6Sk.License:Public Domain: No Known Copyright.License Terms: Standard YouTube license
- Molecular-collisions.Provided by: Wikipedia.Located at: http://en.wikipedia.org/wiki/File:Molecular-collisions.jpg.License:CC By-SA: Attribution-ShareAlike
- Provided past: African Virtual University.Located at: http://oer.avu.org/bitstream/handle/123456789/43/Chemical science%202%xx-%20Introductory%20General.pdf?sequence=6.License:CC By: Attribution
- Arrhenius equation.Provided by: Wikipedia.Located at: http://en.wikipedia.org/wiki/Arrhenius_equation.License:CC By-SA: Attribution-ShareAlike
- Activation free energy, Arrhenius police.Provided by: Steve Lower's Website.Located at: http://www.chem1.com/acad/webtext/dynamics/dynamics-3.html.License:CC Past-SA: Attribution-ShareAlike
- Exponential Decay.Provided by: Wikipedia.Located at: http://en.wikipedia.org/wiki/Exponential%20Decay.License:CC Past-SA: Attribution-ShareAlike
- Endothermic Reaction.Provided past: Wikimedia.Located at: http://eatables.wikimedia.org/wiki/File:Endothermic_Reaction.png.License:CC BY-SA: Attribution-ShareAlike
- OpenStax Higher, Potential, Kinetic, Complimentary, and Activation Energy. Oct sixteen, 2013.Provided by: OpenStax CNX.Located at: http://cnx.org/content/m44425/latest/Figure_06_03_04.jpg.License:CC By: Attribution
- Collision theory explanation.Located at: http://www.youtube.com/watch?5=mBTSwJnZ6Sk.License:Public Domain: No Known Copyright.License Terms: Standard YouTube license
- Molecular-collisions.Provided by: Wikipedia.Located at: http://en.wikipedia.org/wiki/File:Molecular-collisions.jpg.License:CC By-SA: Attribution-ShareAlike
- Provided past: African Virtual University.Located at: http://oer.avu.org/bitstream/handle/123456789/43/Chemistry%202%20-%20Introductory%20General.pdf?sequence=six.License:CC BY: Attribution
- Activated complex.Provided by: Wikipedia.Located at: https://en.wikipedia.org/wiki/Activated_complex.License:CC Past-SA: Attribution-ShareAlike
- Endothermic Reaction.Provided by: Wikimedia.Located at: http://commons.wikimedia.org/wiki/File:Endothermic_Reaction.png.License:CC BY-SA: Attribution-ShareAlike
- OpenStax College, Potential, Kinetic, Free, and Activation Free energy. Oct 16, 2013.Provided past: OpenStax CNX.Located at: http://cnx.org/content/m44425/latest/Figure_06_03_04.jpg.License:CC BY: Attribution
- Collision theory caption.Located at: http://www.youtube.com/picket?five=mBTSwJnZ6Sk.License:Public Domain: No Known Copyright.License Terms: Standard YouTube license
- Molecular-collisions.Provided by: Wikipedia.Located at: http://en.wikipedia.org/wiki/File:Molecular-collisions.jpg.License:CC BY-SA: Attribution-ShareAlike
- Provided past: African Virtual University.Located at: http://oer.avu.org/bitstream/handle/123456789/43/Chemical science%202%twenty-%20Introductory%20General.pdf?sequence=6.License:CC Past: Attribution
This chapter is an accommodation of the chapter "Dimensional Analysis" in Boundless Chemistry by LumenLearning and is licensed under a CC By-SA 4.0 license.
Activation Energy Of Forward Reaction,
Source: https://uen.pressbooks.pub/introductorychemistry/chapter/activation-energy-and-temperature-dependence/
Posted by: thayerwitify.blogspot.com


0 Response to "Activation Energy Of Forward Reaction"
Post a Comment